Release date: 2017-12-13
In the modern medical field, as an important technical means of advanced manufacturing of the "third industrial revolution", bio-3D printing is changing medical means and models to promote medical development and even reshape the medical industry.
The rapid development of 3D printing is also receiving more and more attention from the FDA. In May 2016, the FDA released a draft guide for 3D printing for medical manufacturers, collecting medicines and 3D printing companies such as Johnson & Johnson, American Advanced Medical Technology Association, and Materialise. After a year and a half, the FDA issued a 3D printed medical device manufacturing guidance on December 5, 2017. In addition, the FDA Commissioner stated that the FDA has reviewed more than 100 3D printing devices on the market today, including specific implants and the first 3D printed drug.
Further guidance from the FDA has provided medical device manufacturers with advice on 3D printing technology, as well as what manufacturers need to include when submitting 3D printed medical devices, including various 3D printing methods. Thinking, equipment design, functionality, durability testing, and quality system requirements.
In fact, when the global eye is firmly attracted by the topic of artificial intelligence, the development of 3D printing in the medical field is also worthy of attention.
China's first high-throughput integrated bio 3D printer was successfully developed
On November 23, 2017, scientists from Hangzhou, Beijing, Shanghai, Guangzhou and other places released the first high-throughput integrated bio-3D printer in China. The research results belong to the first year of the 13th Five-Year National Key R&D Program "Development of Functional Materials for Living Devices and High-throughput Integrated 3D Printing Technology Development", representing the top level of bio-based 3D printing equipment in China, and also means The research level in China's related fields has achieved a leap from “running†to “leading†with the international advanced level.
The device integrates more than 50 technological innovations and breakthroughs. The key technology innovation is “Discrete Manufacturing Micro-Tomography (MCT)â€. Theoretically, the imaging depth is not limited, high-resolution, non-contact, and cell-free damage. Online real-time feedback controls printing parameters to achieve non-destructive quality control of 3D printing products, and also provides a new solution for new drug screening, which will promote the creation and development of new drugs in China.
FDA approved several 3D printing products
In June 2017, the FDA approved the first ankle joint 3D printed implant product, iFuse-3DTM, produced by US medical device company SI-BONE, a titanium implant for the treatment of ankle joint disease. Implanted into the human body during minimally invasive surgery for ankle joint disease (SI). The skeletal pore biomimetic structure on the surface of the iFuse-3DTM implant is directly fabricated from a 3D printing device, and the porosity and pore size can be precisely controlled during implant design and fabrication.

iFuse-3DTM 3D Print Ankle Implant
In July 2017, the FDA approved OSSEUS's cervical fusion cage Gemini-C for the treatment of degenerative disc disease. This is a spine implant made of titanium metal and PEEK material using 3D printing technology. It has both a porous titanium structure possessed by a titanium metal implant and a radiation transmission and biology of a PEEK implant. Mechanical properties.
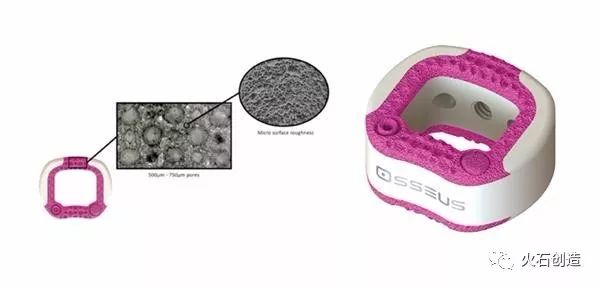
OSSEUS cervical fusion cage Gemini-C
In October 2017, the FDA approved two 3D printed spinal implants, one of which was a 3D printed titanium vertebral implant HAWKEYE Ti from American spine device manufacturer ChoiceSpine LP, and the other was Nexxt. NEXXT MATRIXX 3D printed spinal implant from Spine. NEXXT MATRIXX has powerful radiographic performance and a surface area for bone deposition up to 4 times that of previous spinal implants. The surface texture increases bone cell differentiation and surface osseointegration.

ChoiceSpine's HAWKEYE Ti implant

Nexxt Spine's NEXXT MATRIXX implant
The world's first special 3D printing plastic
The British company Torc2 has developed a composite that can be reshaped at low temperatures. This soft and durable special plastic is solid at 37 ° C (this is also the normal temperature of the human body), at 55 ° C It can become soft and can cause 3D printed medical devices to change shape on the patient's body. For patients with cerebral palsy and hip dysplasia, this plastic can be used to make splints, as well as lower limb prostheses. Remodelable liner.

Stryker funded a customized implant 3D printing project
In October 2017, international orthopaedic equipment giants Stryker and IMCRC (Australian Innovative Manufacturing Cooperative Research Center) funded a total of 14.46 million Australian dollars to support Australian research institutions, universities and hospitals, and jointly launched "Just In Time Implant" The project aims to rapidly manufacture customized implants for patients with bone tumors through 3D printing technology, surgical robots and advanced manufacturing methods, changing the way doctors treat bone tumors and improving patient and healthcare outcomes.
Image giant GPS in the field of 3D printing
At the North American Radiological Society Annual Meeting (RSNA) 2017, Philips introduced its latest version of the comprehensive visualization platform IntelliSpace Portal 10. The software embeds a new 3D modeling application that provides high-performance image analysis solutions with DynaCAD. At the same time, the software is connected to the workflow interface of Stratasys, the world's leading 3D medical device manufacturer. In addition, Philips announced on RSNA that it has partnered with 3D Systems, which introduced a new D2P (DICOM to PRINT) technology that directly converts image data into a digital 3D anatomical model that can be printed directly.
Siemens Medical announced a new collaboration with 3D medical manufacturer Materialise at RSNA to integrate the Materialise Mimics inPrint software for hospital 3D printed anatomical models into its own medical image processing software syngo.via platform for customized personal care And precise medication.
In August 2017, GE Healthcare, through long-term research, combined advanced imaging equipment such as computed tomography (CT) equipment with 3D printing technology, and launched a new AW4.7 Zhihui workstation that is highly compatible with upstream and downstream docking equipment. To achieve efficient medical 3D printing. On December 7th, they signed an exclusive strategic cooperation agreement with Guangyunda (300227) and 3D printing company Stratasys in China to build China's first medical 3D printing overall solution, from medical imaging to 3D modeling to 3D printing. The seamless connection enhances the diagnostic rate of the medical institution and the clinical outcome of the patient.
Source: Flint Creation
Sweeteners refer to Food Additives that can impart sweetness to soft drinks. Sweeteners can be divided into nutritive sweeteners and non-nutritive sweeteners according to their nutritional value; according to their sweetness, they can be divided into low-sweetness sweeteners and high-sweetness sweeteners; according to their source Divided into natural sweeteners and synthetic sweeteners.
Here you can find the related products in Sweeteners, we are professional manufacturer of Sweeteners. We focused on international export product development, production and sales. We have improved quality control processes of Sweeteners to ensure each export qualified product.
Here you can find the related products in Sweeteners, we are professional manufacturer of Sweeteners like D-Mannose, Raffinose, Sucralose, Maltitol, Erythritol, Sorbitol and so on.
D-Mannose, Raffinose, Sucralose, Maltitol, Erythritol, Sorbitol
Xi'an Gawen Biotechnology Co., Ltd , http://www.ahualyn-bio.com