Say goodbye to chemotherapy! New treatments reduce the risk of leukemia progression by 83%
December 13, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, AbbVie and Genentech announced the results of the key phase 3 clinical trial MURANO at the annual meeting of the American Society of Hematology (ASH). The trial showed that Venclexta (venetoclax) + Rituxan (rituximab) significantly reduced the risk of disease progression or death in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) compared with bendamustine + Rituxan by 83%.

Chronic lymphocytic leukemia (CLL) is the most common type of adult leukemia. In 2017, it is estimated that there will be more than 20,000 new cases of CLL in the United States. Although the symptoms of CLL may disappear for a period of time after initial treatment, the disease is considered incurable and many people require additional treatment for cancer recurrence. There are also major medical needs in this area that are not being met.
Venclexta, brought by Abbott and Genentech, is expected to meet this demand. It is a small molecule that selectively binds to and inhibits BCL-2 protein and plays an important role in a process called apoptosis (programmed cell death). Overexpression of BCL-2 protein in CLL is associated with resistance to certain therapies, and blocking BCL-2 restores the cell's signaling system, allowing cancer cells to self-destruct.

The two companies are currently evaluating the efficacy of Venclexta+Rituxan in the treatment of relapsed or refractory CLL and have made good progress. MURANO is a phase 3 open-label, international, multicenter, randomized trial evaluating the efficacy and safety of Venclexta+Rituxan compared to bendamustine + Rituxan in relapsed or refractory CLL. The study enrolled a total of 389 patients who had received 1-3 treatments. Patients were randomized to receive either Venecxta+Rituxan (group A) or bendamustine + Rituxan (group B, hereinafter referred to as control group) in a 1:1 ratio. The primary endpoint of the study was the progression-free survival (PFS) assessed by the investigator. Secondary endpoints included PFS, overall response rate (ORR), and complete response rate (with or without complete blood count recovery) assessed by the Independent Review Board (IRC). CR / CRi), overall survival (OS), minimum residual disease (MRD) status, duration of remission, event-free survival, and time to next CLL treatment.
The results show:
• Patients treated with Venclexta+Rituxan had significantly longer PFS as assessed by the investigator compared with controls (HR=0.17; 95% CI 0.11-0.25; p<0.0001; median PFS did not reach vs. 17.0 months).
o Two years later, 84.9% of patients in the Venclexta+Rituxan group did not experience disease progression, compared with 36.3% of the control group who did not experience disease progression.
o Consistent benefits were observed in all patient subgroups of Venclexta+Rituxan compared to the control group, including high-risk and low-risk groups.
o IRC assessment was consistent: Venclexta+Rituxan reduced disease risk or mortality by 81% compared with controls (HR=0.19; 95% CI 0.13-0.28; p<0.0001)
• Clinical benefit of Venclexta+Rituxan was consistent with key secondary endpoints compared with controls, including OS (HR=0.48; 95% CI 0.25-0.90; median not met), ORR assessed by the investigator (93.3%) Vs. 67.7%) and CR/CRi (26.8% vs. 8.2%). These results were not statistically significant. In addition, Venclexta+Rituxan observed a higher MRD negative rate (83.5% vs. 23.1%) at all times compared to the control group. (MRD negative is defined as less than 1 CLL cell in 10,000 leukocytes.)
• No new safety issues were observed in the Venclexta+Rituxan treatment portfolio.
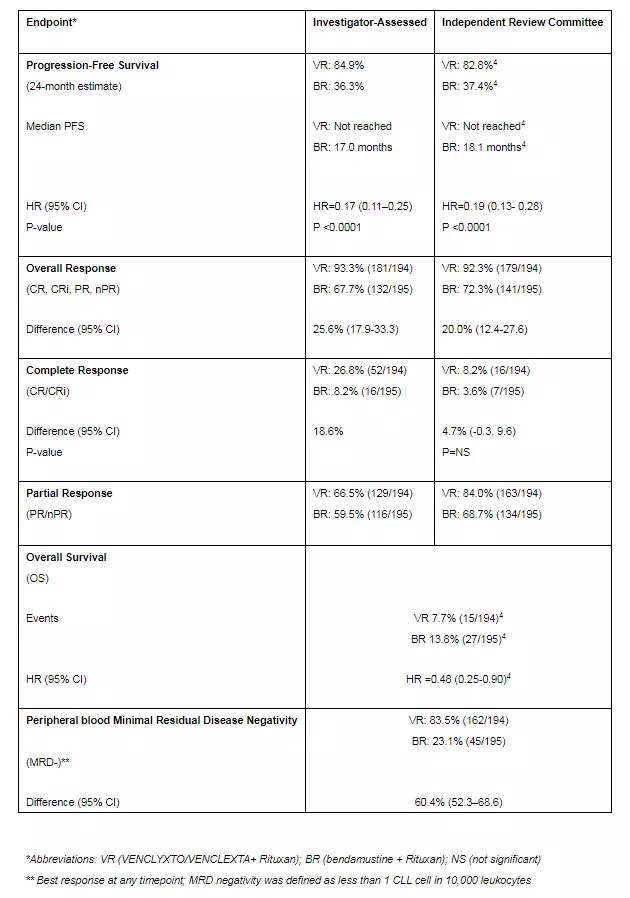
â–² Complete data for the study (Source: Aibowei)
“The results of the MURANO study suggest that Venclexta plus Rituxan may provide an important new chemotherapy-free regimen for patients with previously treated chronic lymphocytic leukemia,†said Dr. Sandra Horning, Chief Medical Officer and Head of Global Product Development at Genentech. We are particularly encouraged by the usefulness of the key efficacy measures observed compared to current standard treatments. We look forward to discussing these results with health regulators."
"According to the efficacy and safety of the trial, the combination of Venclexta and Rituxan may offer a new chemotherapy-free regimen for patients with relapsed/refractory CLL," said Dr. John Seymour, Principal Investigator of the MURANO trial: "We will continue to monitor Test patient safety and efficacy to get more data and information."
We expect this new non-chemotherapy regimen to bring new treatment options to patients with CLL as soon as possible.
Reference materials:
[1] AbbVie, Genentech's Venclexta Plus Rituxan Crushes Chemo in Rituxan CLL Study
[2] Genentech Official Website
[3] AbbVie Official Website
Frozen squid whole refers to squid that has been cleaned, gutted, and frozen whole, including the head, body, and tentacles. This type of squid is commonly used in various cuisines, including Asian, Mediterranean, and Italian, and can be prepared in many ways, such as grilling, frying, or boiling. Frozen squid whole is often sold in seafood markets, grocery stores, and online retailers and can be stored in the freezer for several months.
Frozen Squid Whole,Frozen Boneless Squid,Frozen Giants Squid Frozen Seafood,Frozen Seafood Frozen Squid
Zhoushan Fudan Tourism CO., LTD , https://www.fudanfood.com