The bleeding rate is reduced by 88%, and the latest data on new hemophilia drugs is released.
December 13, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Genentech announced the latest data on Hemlibra (emicizumab-kxwh), a new hemophilia drug, at the annual meeting of the American College of Hematology (ASH). Long-term data showed that many patients did not see bleeding after treatment with Hemlibra compared to previous prevention or BPA (Bypassing Agent) therapy.

Hemophilia A is a serious genetic disease in which the patient's blood cannot coagulate normally, resulting in uncontrolled spontaneous bleeding. Hemophilia A affects approximately 320,000 people worldwide, and 50-60% of them have a serious form of the disease. Patients with hemophilia A lack a coagulation protein called factor VIII. At the same time, a serious complication of hemophilia A is that the patient develops an inhibitor of factor VIII replacement therapy, which is an antibody developed by the body's immune system that binds and blocks the replacement factor VIII, making it impossible. A level sufficient to control bleeding. Most patients with hemophilia A develop a factor VIII inhibitor, intermittent or prophylactic infusion of Bypassing Agent (BPA) to control bleeding. This patient population also has huge medical needs that are not being met.
Hemlibra is a bispecific factor IXa and a factor X directed antibody. It aggregates the proteins needed to activate the natural coagulation cascade, Factor IXa and Factor X, to restore the clotting process in patients with hemophilia A. Hemlibra is a prophylactic treatment that can be administered by subcutaneous injection of a ready-to-use solution once a week. It is expected to provide new treatment options for patients with hemophilia A who develop factor VIII inhibitors. The drug was approved by the US FDA in November 2017.
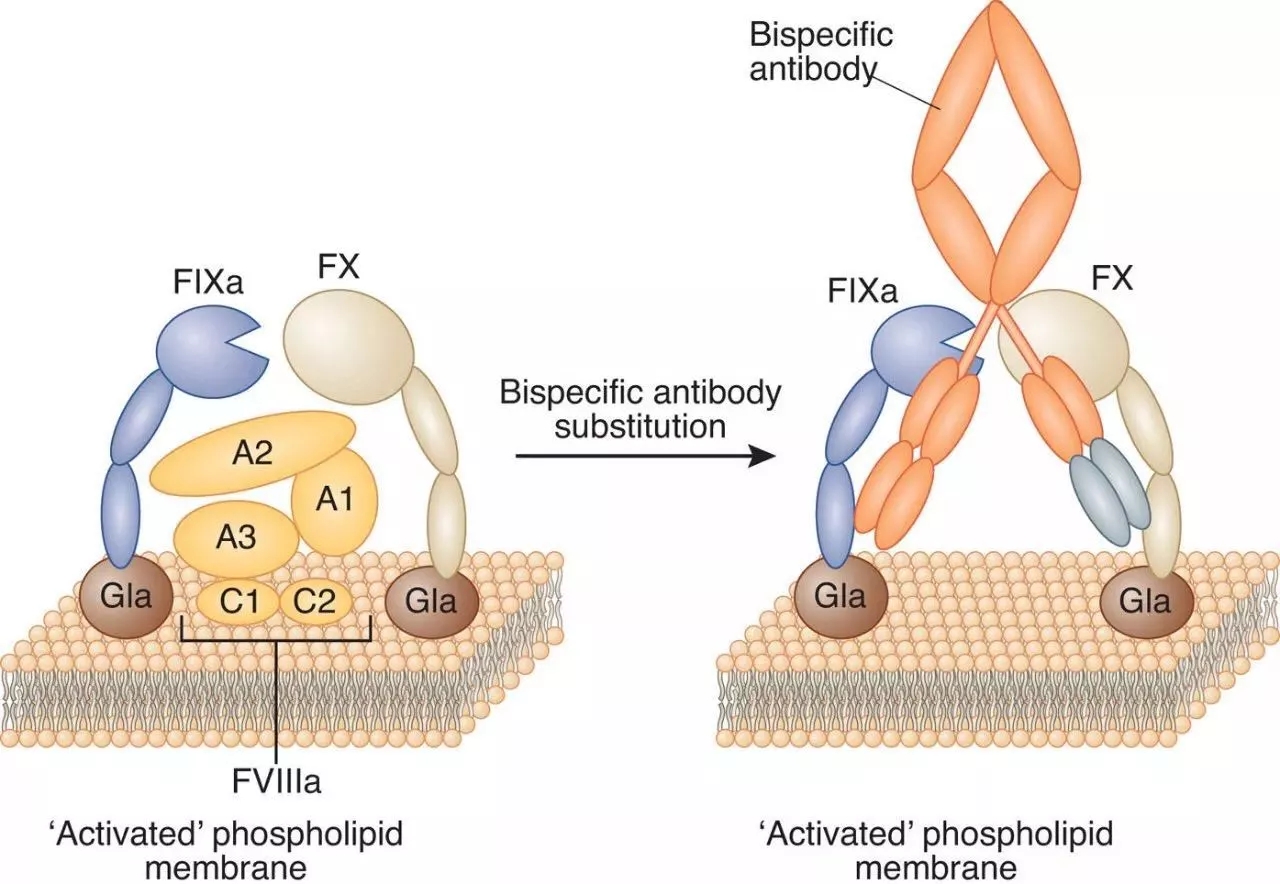
â–²The mechanism of action of bispecific factor IXa and factor X directed antibodies (Source: Nature)
Genentech's medical director and global research and development director, Dr. Gallia Levy, who is responsible for the development of Hemlibra, shared the new data and described the feasibility of treating hemophiliacs with factor VIII inhibitors. Long-term data showed that many patients did not see bleeding after treatment with Hemlibra compared to previous prevention or BPA therapy.
In addition, Genentech also disclosed long-term data from early Haven 1 and Haven 2 clinical trials, which showed that Hemlibra's efficacy for patients can last for a long time. Data from the Haven 2 trial showed that 94.7% of children with hemophilia A who carried inhibitors did not see bleeding after receiving Hemlibra. According to the latest data from the Haven 1 trial, adult and adolescent patients have a 88% reduction in bleeding rates after treatment.

Genentech also published mid-term data from the Haven 4 trial at the ASH conference. The results showed that bleeding was effectively controlled in patients who received treatment every four weeks after a median of 17 weeks of treatment.
Dr. Levy said that Hemlibra can really change the lives of people with hemophilia who carry factor VIII inhibitors. In the past, these patients have been difficult to be truly cured, which means their needs have not been met. “We learned from patients that they have significantly improved their quality of life. For these special patients, they have been looking forward to such a new drug for a long time,†Dr. Levy said.
We hope that this new hemophilia drug will bring effective treatment options to patients and families as needed, and ease the burden of treatment.
Reference materials:
[1] Genentech's Hemlibra Data Continues to Wow at ASH
[2] Genentech's official website
Shelled shrimps,Dried shrimps,Frozen Bamboo shrimp,Pandalus borealis,Red Shrimp,Coldwater Shrimp
Zhejiang Ocean Family Co., Ltd., , https://www.ocean-family.com