Of Human Pluripotent Stem Cells
2 Eppendorf AG, Hamburg, Germany
Abstract
Since the groundbreaking discovery of human induced pluripotent stem cells (hiPSCs) by Shinya Yamanaka in 2006, the use of pluripotent stem cells (PSCs) has experienced a boom, thus representing an important tool for biological research. The ultimate goal during the cultivation of PSCs The these of their pluripotency, routinely characterized by their morphology, growth, pluripotency marker expression, as well as by their differentiation potential. The selection of a defned culture system consisting of a growth surface and culture medium is therefore crucial. Coating materials for PSC expansion, based on feeder layers or animal-derived protein mixtures, present a non-defned growth surface, which poses an obstacle when aiming at applications requiring high consistency.
The ready-to-use Eppendorf CCCadvancedTM FN1 motifs surface offers a comfortable alternative for the long-term cultivation of PSCs in a feeder-free as well as animal- and human-component-free environment. This novel surface is made up of fbronectin - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
The FN1 motifs surface combines contrast with reliable PSC performance: the ready-to-use consumable signifcantly reduces labor time and effort for scientists while offering a fully synthetic culture system with a high level of consistency during long-term PSC expansion.
Introduction
Due to their extensive in vitro self-renewal properties and their ability to differentiate into all three constituent germ layer cell types, PSCs, and especially hiPSCs, offer consid erable and exciting promises in a wide range of cell applic cations [1, 2]. As a critical but essential milestone to fully exploit the potential of these stem cells, PSC expansion conditions have progressively moved from the traditional mouse embryonic fbroblast (MEF) feeder layer-based cul ture system towards more defned feeder-free cell culture systems [3, 4].
The common common feeder-free alternatives are still based on the use of complex animal-derived protein mixtures presenting a non-defned composition, variable lot-to-lot quality and purity as well as a potential pathogen contamina-tion risk. Standard biological coating types, for example Corning® Matrigel®, are enriched with unde fned growth factors and extracellular matrix (ECM) compo nents known to sustain cell adhesion and pluripotency [5]. To ensure robust cell performances in downstream applica tions, consistent and Defened culture conditions are crucial. In this context, fully synthetic, animal-component-free culture systems are of great interest [6, 7, 8].
Based on a proprietary coating technology, the Eppendorf CCCadvanced FN1 motifs surface is made up of synthetic fbronectin-derived motifs (including RGD), specifcally designed to mimic the cell attachment site of native ECM proteins. Used in combination with a well-defned culture medium And dissociation solution, this surface represents an effective animal- and human-component free alternative, not only to the conventional feeder layer-based culture system, but also to other culture systems that depend on biological coating. Being ready-to-use, it The FN1 motifs surface is highly suitable for the long-term expansion Of undifferentiated hiPSCs. Throughout at least 20 successive passages, hiPSCs maintain their typical cell morphology, stable doubling time and karyoty Pp as well as pluripotent marker expression, when cultured on this surface. The in vitro trilineage differen tiation capacity provides defnitive proof of the preservation of the functional pluripotency of these cells.
Materials and Methods
Cell culture conditions and surface transition
Cryopreserved hiPSCs (SC102A-1, SBITM, USA) were initially thawed and pre-cultivated on a Corning Matrigel-coated surface (Corning, USA) in a xeno-free culture medium specifcally adapted for feeder-free hiPSC expansion (Gibco® Essential 8TM, Thermo Fisher Scientifc®, USA) according to manufacturer's recommendations. After 5 passages on the Corning Matrigel-coated surface, the cells were seeded on the Eppendorf CCCadvanced FN1 motifs surface using the classic clump passage procedure. Briefly, cell clumps were harvested Using Gentle-StemTM Enzyme-Free Human ESC/ iPSC Dissociation Solution (SBI, USA) according to manu facturer's recommendations. The cell clump suspension was plated directly into a ready-to-use FN1 motifs 6-well plate and incubated under standard cell Culture conditions (37 °C, 5% CO 2, humidifed atmosphere).
Starting at 24 h post-seeding, 100% of the culture medium was replaced with fresh medium daily until the confluency level of interest was reached. Cells were maintained on the FN1 motifs surface during 25 successive passages. Microscopic visual assessment was performed daily to In parallel, hiPSCs were cultivated on the Corning Matrigel coated surface as well as the Corning® Synthemax® II-SC Self-Coating Substrate (Corning, USA) In order to compare hiPSC growth performances on Eppendorf CCCadvanced FN1 motifs with those obtained on a biological feeder-free culture system and a competitor synthetic surface, respec tively.
Cell growth evaluation
Cell growth and viability were assessed at each passage through cell counting performed on 3 independent wells. After complete cell detachment using Accutase® (Merck MilliporeTM, Germany), the single cell suspension obtained through gentle pipetting was transferred to a tube. Rupture with D-PBS to recover any remaining cells. A cell count was performed on each homogenized cell suspension using the Vi-CELLTM automated cell counting device (Beckman Coulter®, USA). Population doubling (PD) and doubling time (DT) We are calculating using the respective formula:
PD = (log10(NH)-log10(Ni))/log10(2)
DT = time in culture (hours) x (LN(2)/ LN(NH/Ni))
NH = total number of harvested viable cells
Ni = initial number of seeded cells
Stability of doubling time development has been compared using Fisher test for equality of variances.
Alkaline Phosphatase (ALP) staining
The Red-ColorTM AP Staining Kit (SBI, USA) was used at dif ferent time points during the long-term expansion process in accordance with the manufacturer's recommendations. Pluripotent marker expression assessment using immunostaining
The preservation of a high expression level of several key pluripotent markers was demonstrated by immunostaining at the beginning and at the end of the hiPSCs expansion process using the Pluripotent Stem Cell 4-Marker Immuno cytochemistry Kit (Thermo Fisher Scientifc, USA) accord ing to the This kit allows specifc fluorescent staining of two specifc nuclear markers (OCT4 and SOX2) and two specifc cell surface markers (SSEA4 and TRA-1-60) in cells counterstained with NucBlue® Fixed Cell Stain (DAPI nuclear DNA stain). Fluorescent stained cells were observed with the Invitrogen® EVOS® FL Cell Imaging System (Thermo Fisher Scientifc, USA).
Pluripotent marker expression assessment using flow cytometry analysis
The control of the expression level of 3 key pluripotent markers (Nanog, OCT3/4 and SOX2) was performed through flow cytometry analyses using the BDTM Pluripotent Stem Cell Transcription Factor Analysis Kit (BD Biosciences, USA). At the end of the expansion Process, viable cell density was determined from single-cell suspensions by cell count. The appropriate number of cells (input cells: 10,000) was prepared for FACS analysis according to the manufacturer's recommendations. For each cell type analyzed, a sample of unstained cells and An isotype control were prepared in or der to measure autofluorescence and non-specifc staining, respectively. Cells were analyzed with a BD FACSVerseTM flow cytometer (BD Biosciences, USA), and data analysis was performed using the BD FACSUITETM SOFTWARE (BD Biosciences , USA).
Trilineage differentiation potential
To confrm the ability of expanded hiPSCs to differentiate into cells of each of the three embryonic germ layers (en doderm, mesoderm and ectoderm), an in vitro non-directed differentiation process was performed. Briefly, after expan sion, cells were frst used to Form embryoid bodies (EBs) in suspension containing an EB culture medium without bFGF (basic Fibroblast Growth Factor) before transfer to a Corning Ultra-Low Attachment vessel (Corning, USA).
EB formation required 4 days of incubation at 37 °C and 5% CO 2 with daily 100% volume medium refreshment.
After 4 days in suspension, these EBs were seeded on the FN1 motifs surface and allowed to spontaneously differentiate over 14 days in the same culture medium, with medium refreshments performed every two days. Finally, the differenti ated cells obtained were characterized by immunostaining using The 3-Germ Layer Immunocyto-chemistry Kit (Thermo Fisher Scientifc, USA), allowing specifc fluorescent staining of well-established markers characteristic of the three embry onic germ layers (β-III tubulin (TUJ1)) for ectoderm, smooth muscle actin ( SMA) for meso-derm, and alpha-fetoprotein (AFP) for endoderm) in cells counterstained with NucBlue Fixed Cell Stain (DAPI nuclear DNA stain). This kit was used according to the manufacturer's recommendations, and fluorescent-stained cells were visualized using The Invitrogen EVOS FL Cell Imaging System (Thermo Fisher Scientifc, USA).
Karyotype analysis
Karyotype analysis was performed on fxed metaphas blocked cell samples. In order to arrest the cell cycle in metaphase, cells were incubated for 50 minutes at 37 °C in the presence of KaryoMAX® Colcemid® (Thermo Fisher Scientifc, USA). After harvesting and Hypotonic treatment, swollen cells were carefully fxed. Fixed cell suspensions were then sent to an external service (Cell Guidance Systems Ltd, UK) for G-banding (Giemsa-banding) karyotype analysis. For each sample, 17 G-banded metaphase spreads were Created and analyzed.
Results and Discussion
Robust long-term xeno-free expansion of undifferentiated hiPSCs on the Eppendorf CCCadvancedTM FN1 motifs surface
The hiPSC morphology was monitored daily during the entire expansion process of 25 successive passages on the FN1 motifs surface (Figure 1). Their morphology corresponds to the hiPSC morphology expected on a feeder-free culture system, characterized by compact cells with a high nuclear to -cytoplasm ratio and a prominent nucleolus, forming flat, tightly packed, shiny colonies with well-defned boarders [9]. This morphology remained stable across all passages and it was similar to the morphology of hiPSCs expanded on the Corning Matrigel-coated surface used As a reference feeder free culture system.

Figure 1: hiPSCs morphology during long-term expansion
hiPSCs cultured on the Eppendorf CCCadvanced FN1 motifs surface as well as on Corning Matrigel-coated surface showed significant homogenous flat and shiny colonies with well-defned borders. The images showed representative areas at passage numbers 9 and 24, respectively. Scale bar indicates 400 Mm.
The FN1 motifs surface supports a linear cumula-tive popula tion doubling curve during long-term expansion (Figure 2). Between two successive passages, surface colony coverage levels of 80-90% were obtained within approximately 4 days. An efcient and stable hiPSC Doubling time was measured at an average value of 24 h. This doubling time was similar to that of hiPSCs expanded on a Corning Matrigel-coated sur face, indicating that cells expanded on the FN1 motifs surface present the same proliferation rate as cells expanded on the Additionally, hiP SCs expanded on the FN1 motifs surface exhibit a signifcant ly more stable doubling time compared to cells expanded on a synthetic self-coating competitor surface, Corning Synthemax II-SC, as confrmed by the F-test For equality of variances.
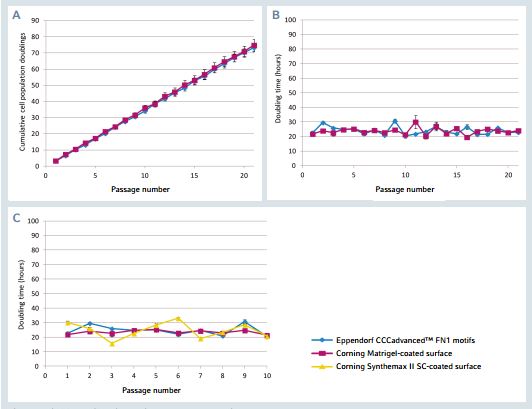
Figure 2: hiPSCs amplification during long-term expansion
A. Cumulative cell population doublings for hiPSCs cultured on the FN1 motifs surface as well as on the Matrigel-coated surface for 20 successive passages. B. Cell population doubling time for hiPSCs cultured on the FN1 motifs surface as well as on the Matrigel-coated Surface for 20 successive passages. C. Cell population doubling time for hiPSCs cultured on the FN1 motifs surface, the Matrigel-coated surface as well as on the Synthemax II-SC-coated surface for 10 successive passages. The results represent a mean of three Independent wells.
hiPSCs expanded on Eppendorf CCCadvancedTM FN1 motifs surface maintain a functional pluripotency
To ensure that fundamental properties of undifferentiated hiPSCs expanded in this new well-defned culture system have not changed, extensive pluripotent cell characterizations were performed. During the entire expansion process, very low levels of spontaneous cell differentiation were observed for hiPSCs expanded on the FN1 motifs These observations, initially based on cell morphology, were confrmed by high level expression and activity of a specifc alkaline phosphatase (ALP) enzyme isoform present in pluripotent stem cells. hiPSCs expanded on the FN1 motifs Surface exhibit a high and stable level of ALP expression/activity during the entire expansion pro cess (Figure 3). Moreover, the ALP expression/activity high lighted was similar to that observed for hiPSCs expanded on the biological Corning Matrigel-coated surface.
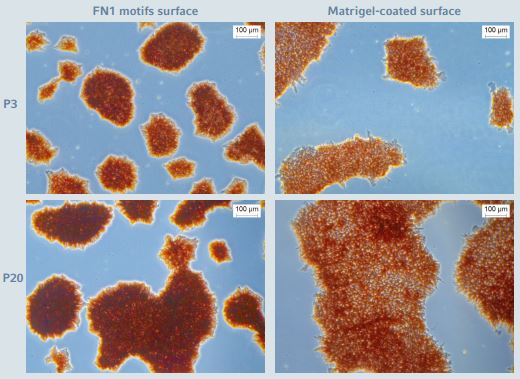
Figure 3: Alkaline phosphase staining during long-term expansion of hiPSCs
hiPSCs cultured on the CCCadvanced FN1 motifs surface as well as on Corning Matrigel-coated surface showed high and stable levels of ALP expression. The images show representative areas at passage numbers 3 and 20, respectively. Scale bar indicates 100 μm.
In order to complete the qualitative data set and to confrm the preservation of the cell pluripotency status after long term expansion on the FN1 motifs surface, the expression of several other key self-renewal markers was assessed using immunostaining and flow cytometry analysis. After 25 successive Passages on the FN1 motifs surface, hiPSCs continued to exhibit a high expression level of two pluripotent specifc surface proteins, TRA-1-60 and SSEA4, and of two self-renewal-associated nuclear transcription factor proteins, SOX2 and OCT4 (Figure 4) .
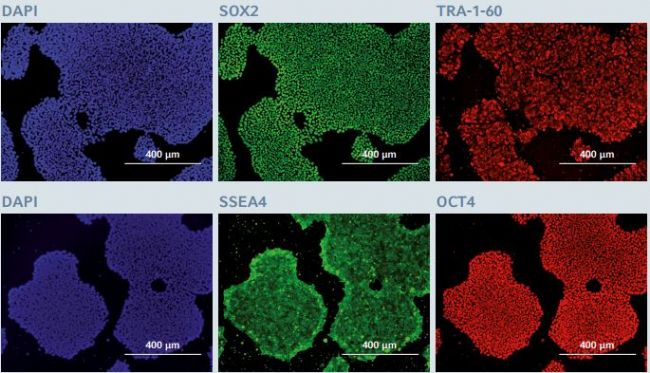
Figure 4: Immunofluorescence staining of pluripotency markers after long-term expansion of hiPSCs
After 25 successive passages on the CCCadvanced FN1 motifs surface, hiPSCs expressed the pluripotency markers SOX2, TRA-1-60, SSEA4 and OCT4, as evaluated through immunofluorescent staining. Cells were counterstained with a classic nuclear marker, DAPI. The images show representative areas Of hiPSCs stained after 25 passages in culture. Scale bar indicates 400 μm.
Flow cytometry analysis of the quantitative expression of 3 key pluripotency-associated transcription factors (Nanog, OCT3/4 and SOX2) complemented the image-based analysis (Figure 5). Even after long-term expansion on the FN1 motifs surface, hiPSCs still exhibit a high level of Nanog, OCT3/4 and SOX2 expression (> 95% positive cells in the entire population). The pluripotency marker expression profle was similar to that observed in hiPSCs expanded on the Corning Matrigel-coated surface.
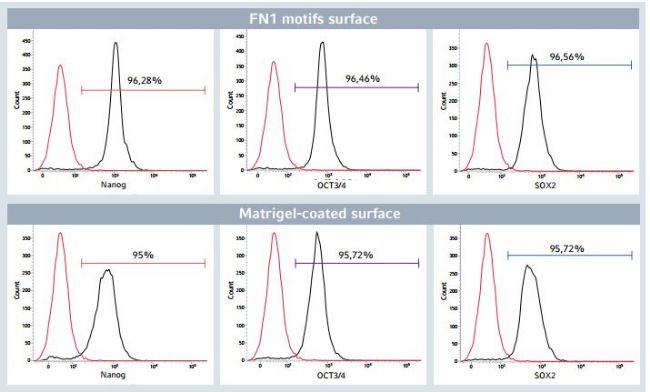
Figure 5: Flow cytometry analysis of pluripotency markers after long-term expansion of hiPSCs
After 24 successive passages on the Eppendorf CCCadvanced FN1 motifs surface, > 95% of the total hiPSC population expressed the pluripotency markers Nanog, OCT3/4 and SOX2, as evaluated by flow cytometry analysis. This pluripotency marker expression profle was comparable to hiPSCs cultured on Isotype controls validated the specifcity of the stain. Unstained cells (indicated in red) determined the percentage of positive cells for the three markers of interest (indicated in black).
The functional pluripotency of hiPSCs after long-term expansion on the FN1 motifs surface evaluated by their in vitro differentiation into cells of the three embryonic germ layers: endoderm, mesoderm and ectoderm (Figure 6). As suggested by positive specifc staining of well- Established markers of the three embryonic germ layers, hiPSCs preserved their trilineage differentiation potential even after 20 successive passages on the FN1 motifs surface.

Figure 6: Trilineage differentiation potential after long-term expansion of hiPSCs
After 20 successive passages on the Eppendorf CCCadvanced FN1 motifs surface, hiPSCs maintained their trilineage differentiation potential, as examined by specifc fluorescent staining of three specifc embryogenic germ layer markers: β-III tubulin (TUJ1) in ectoderm, alpha-fetoprotein (AFP) in Endoderm and smooth muscle actin (SMA) in mesoderm. Cells were counterstained with a classic nuclear marker, DAPI. Scale bar indicates 400 μm.
Genomic stability after long-term xeno-free expansion on the Eppendorf CCCadvancedTM FN1 motifs surface
Long-term expansion of PSCs under feeder-free conditions can be responsible for the occurrence of chromosomal ab normalities [10]. In order to monitor the genomic integrity of hiPSCs after long-term expansion on the FN1 motifs surface in xeno-free culture medium , a G-banding karyotype analysis was performed on fxed metaphase-blocked cell samples obtained after 20 successive passages. Expanded hiPSCs maintained a normal human karyotype (46XY) without chromosomal abnormalities, thus confrming the genomic stability of hiPSCs after long-term expansion on the FN1 motifs surface (Figure 7).

Figure 7: Karyotype analysis after long-term expansion of hiPSCs
After 20 successive passages on the Eppendorf CCCadvanced FN1 motifs surface, hiPSCs exhibit a normal G-banding karyotype without any chromosomal aberrations (46XY).
Conclusion
The ready-to-use Eppendorf CCCadvanced FN1 motifs surface supports efcient, long-term hiPSC expansion in a defned, animal- and human-component-free culture system. Its use is associated with a consistent and robust growth rate of hiPSCs displaying their Characteristic morphology.
During the expansion process across at least 20 successive passages on the FN1 motifs surface, hiPSCs remain undiffer entiated and retain all pluripotent stem cell-specifc features, including the trilineage differentiation potential, as well as their genomic integrity.
Literature
[1] Kumar D, Anand T, Kues WA. Clinical potential of human-induced pluripotent stem cells: Perspectives of induced pluripotent stem cells. Cell Biology and Toxicology 2017; 33(2): 99-112.
[2] Bilic J, Izpisua Belomonte JC. Concise review: induced pluripotent stem cells versus embryonic stem cells: close enough or yet too far apart? Stem cells 2012; 30(1): 33-41.
[3] Celiz AD, Smith JG, Langer R, Anderson DG, Winkler DA, Barrett DA, Davies MC, Young LE, Denning C, Alexander MR. Materials for stem cell factories of the future. Nature Materials 2014; 13(6) : 570-579.
[4] Villa-Diaz LG, Pacut C, Slawny NA, Ding J, O'Shea KS, Smith GD. Analysis of the factors that limit the ability of feeder cells to maintain the undifferentiated state of human embryonic stem cells. Stem Cells Development 2009; 18(4): 641-651.
[5] Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology 2001; 19(10): 971-974.
[6] Tong Z, Solanki A, Hamilos A, Levy O, Wen K, Yin X, Karp JM. Application of biomaterials to advance induced pluripotent stem cell research and therapy. EMBO 2015; 34(8): 987-1008.
[7] Villa-Diaz LG, Nandivada H, Ding J, Nogueira-de-Souza NC, Krebsbach PH, O'Shea KS, Lahann J, Smith GD. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Biotechnology 2010; 28(6): 581-583.
[8] Yang K, Lee J, Cho SW. Engineering biomaterials for feeder-free maintenance of human pluripotent stem cells. International Journal of Stem Cells 2012; 5(1): 1-5.
[9] Lambshead JW, Meagher L, O'Brien C, Laslett AL. Defning synthetic surfaces for human pluripotent stem cell culture. Cell Regeneration 2013; 2(1): 7.
[10] Rosler ES, Fisk J, Ares X, Irving J, Miura T, Rao MS, Carpenter MK. Long-term culture of human embryonic stem cells in feeder-free conditions. Developmental Dynamics 2004; 229(2): 259- 274.
Product That Can Be Eaten Straight Away
Coffee Ice Cream Crunch Cone,Extra Large Waffle Cones,Purple Potato Wafer Cone,Square Wafer Ice Cream Tart
Tianjin Yongkang Food Co., Ltd , https://www.yongkangfood.com