The reporter learned from the press conference that the review and approval of drug re-registration is in full swing.
Article 42 of the “Regulations on the Implementation of the Drug Administration Law†stipulates: “The drug approval number issued by the drug regulatory authority under the State Council, the “Import Drug Registration Certificate†and the “Pharmaceutical Product Registration Certificate†are valid for 5 years. If the production or import continues, it shall apply for re-registration 6 months before the expiration of the validity period. When the drug is re-registered, relevant information shall be submitted in accordance with the provisions of the drug regulatory authority under the State Council. The re-registration of the drug is the listed drug established by relevant laws and regulations. One of the means of supervision is the statutory duties of the food and drug regulatory authorities.
It should be said that re-registration of drugs is a routine task in drug registration management. However, this re-registration is one of the special tasks in the “Notice of the General Office of the State Council on the National Action Plan for Rectifying and Regulating the Drug Market Orderâ€, and the drug re-registration must be checked with the drug approval number, and the drug production process and prescription. Verification and other work are combined, so it can also be said that the drug re-registration work is an in-depth and continuation of the special rectification work.
In August this year, the Food and Drug Administration issued the "Notice on Doing a Good Job in the Examination and Approval of Drug Re-registration", which clarified the "Review Points for Drug Re-registration" and initiated the examination and approval of drug re-registration.
Re-registration of drugs is a review of the conditions for applying for re-registered varieties, the performance of statutory duties by the manufacturer, and the requirements for continued approval of the drug approval documents. As long as the work specified in the regulations and drug approval documents is completed within the validity period of the approval document, products that have no major problems during the period can be re-registered and approved in theory. However, if the conditions for re-registration of the drug are not met, it is resolutely not re-registered, and the approval document number will be cancelled after the expiration of the validity period of the approval document.
The re-registration of the drug set up 12 review points and clarified the review and approval requirements. If any of these conditions are not re-registered, they will not be re-registered. By carrying out the drug re-registration work, the varieties that do not have the production conditions, the quality is not guaranteed, and the safety risks are high will be eliminated. According to the requirements, until September 30 next year, all the varieties that have expired the approval documents must be re-registered, and the varieties that have not been approved for re-registration will not be available for sale. The re-registration of drugs is mainly undertaken by the provincial drug regulatory authorities.
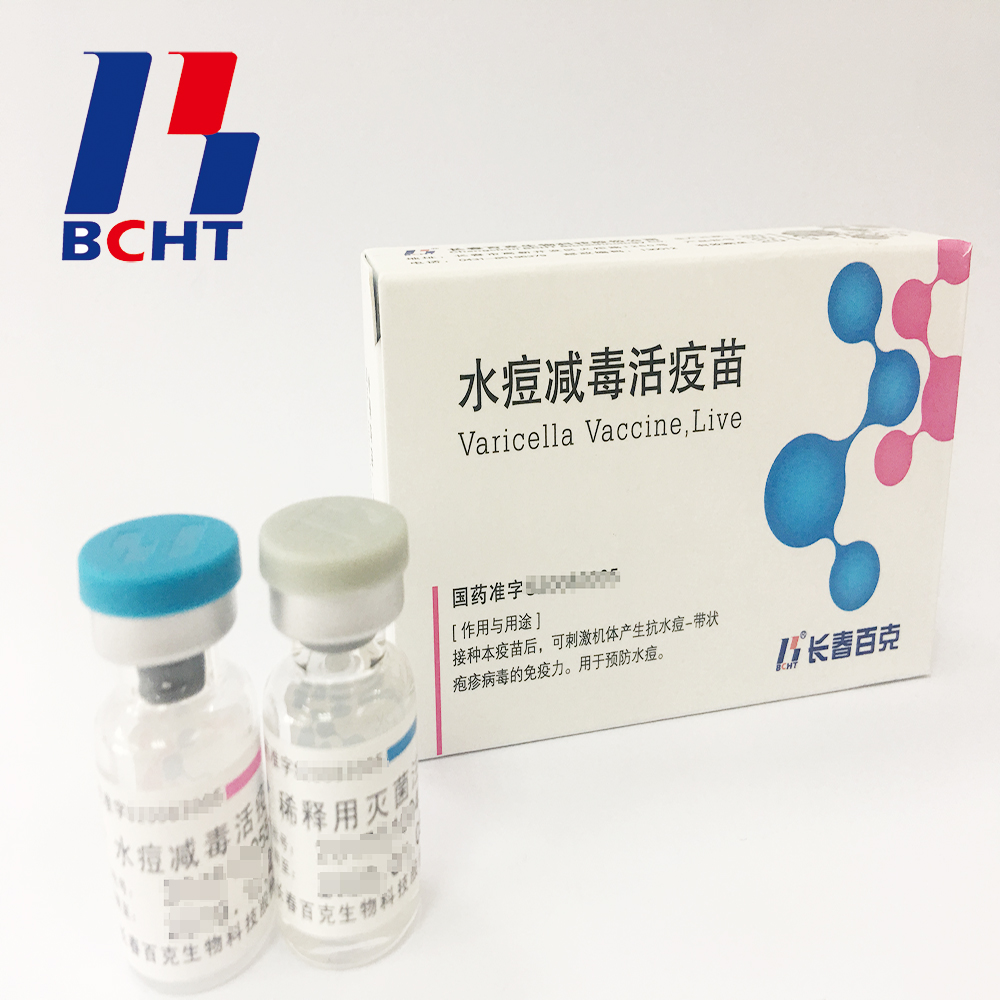
Bulk of varicella vaccine,semi-Finished Products.It has good safety with low ratio in adverse reaction. And it has been exported to Bangladesh.
In 2008, BCHT successfully launched its product Varicella Vaccine, Live in China. In the following years, BCHT continuously committed to improving the product and led in 2010 removal of gelatin from adjuvant and extended the vaccine shelf-life up to 36 months which is the longest one in the world in 2011. And it has the following qualities.
Biotechnology Bioproducts Preventive,Final Bulk Medicine Preventive,Final Bulk Pharmaceutical Preventive,Vaccine Biological Products Preventive
Changchun BCHT Biotechnology Co. , https://www.ccbcht.com