Release date: 2014-02-25
I. Event traceability On February 9, 2014, the State Food and Drug Administration and the National Health and Family Planning Commission jointly issued a notice: “General Office of the General Administration of Food and Drug Administration, National Health and Family Planning Commission Office on Strengthening Clinical Use of Gene Sequencing Related Products and Technologies Management Notice (hereinafter referred to as “Enhanced Supervision Noticeâ€), the notice requires that any medical institution may not carry out clinical application of gene sequencing before the relevant access standards and management regulations are promulgated, and those that have already been carried out shall stop immediately. If the notice continues to be carried out after the issuance, the local health administrative department shall investigate and deal with it according to the law. After the extensive dissemination and interpretation of the "Regulations on Strengthening Supervision", it caused a violent reaction in the industry. The author believes that in order to better understand and interpret the "Enhanced Supervision Notice", the notice should be interpreted in conjunction with a number of guidance documents issued by the CFDA (within 30 days), respectively: January 14, 2014, CFDA released Notice of the General Office of the Food and Drug Administration on the Definition of Three Product Categories, such as Genetic Analyzers (hereinafter referred to as the “Classification Definition Noticeâ€), the “Classification Definition Notice†stipulates that the genetic analyzer is managed according to Class III medical devices, through the National Bureau. Approval registration; sequencing reaction universal kit according to Class I medical device management; aneuploidy sequencing algorithm software (prenatal screening) depending on the situation, if the software only uses general function calculation, not according to medical device management; if used Enterprise-specific algorithms are managed as Class II medical devices. The Classification Definition Notice provides a detailed description of the registration of genetic sequencing related instruments, and detailed regulations on the management of prenatal screening related algorithm software. The author believes that the introduction of the Classification Definition Notice is a precursor to CFDA's strict registration management of the gene sequencing industry (including non-invasive prenatal screening). This notification serves as a guiding document for the registration of related instrument reagents and supporting algorithm software, and gene sequencing. Relevant enterprises in the industry should use this notice as a guideline to carry out the declaration of related products, so that mature sequencing-related clinical products can truly jump out of the gray area and operate in the sun. On February 7, 2014, CFDA issued the Notice of the General Administration of Food and Drug Administration on Printing and Distributing the Special Approval Procedure for Innovative Medical Devices (Trial) (hereinafter referred to as the “Special Approval Noticeâ€), as specified in the “Special Approval Notice†since March 2014. From the 1st, the food and drug supervision department will conduct the review and approval of the medical devices (both domestic and overseas applicants) that meet the corresponding conditions. For the registration of innovative medical devices, the provincial bureau 20 working days and the national bureau must respond within 40 working days. Experts interpret: If you follow the special approval and there is no relevant problem, it only takes 60 working days to enter the public notice. The minimum publicity period is 10 working days. In general, from the time of filing to registration, it is possible to complete it in about 15 weeks, which is much smaller than before. For the genetic analyzers involved in the gene sequencing industry, if the approval process is approved through special approval procedures, the approval time will be greatly shortened, and the official approval time of relevant clinical products will be significantly advanced, which can be interpreted as one of the industry's positive. On February 9th, 2014, the two ministries jointly issued the “Regulations on Strengthening Supervisionâ€, and the public opinion was widely concerned, and the companies in the industry also responded urgently: On February 15, 2014, Huada Gene announced that the non-invasive prenatal screening project was received After the regulatory notice, Huada Gene submitted the relevant documents to CFDA for the first time (the industry believes that its related products will be approved according to the Special Approval Notice), including the sequencer, related reagents and analysis software. The sequencer used for the declaration is a sequencer that was acquired by CG before the acquisition of CG. It is called BGISEQ. However, some insiders believe that the technical indicators such as sequencing flux of BGASEQ's own BGISEQ platform cannot meet the needs of existing products. Huada is more concerned with the first "license" for non-invasive prenatal screening. Berry and Kang, and Annoyouda, who also cultivated in the field of non-invasive prenatal screening, are the “Hua Xiaoshi†in the background of Huada University. The momentum is fierce. They all use Illumina’s Hiseq2000/2500 platform, although they are not official. The news, but the author believes that its sequencing platform and related algorithm software declaration registration work has been carried out. Daan Gene (Guangzhou Aijian) also wants to get a share in this field. Based on a good relationship with LifeTech, they are reporting with LifeTech's Proton... II. Event Analysis and Interpretation In the “Regulations on Strengthening Supervisionâ€, “prenatal gene sequencing†was mentioned twice, pointing to the most mature non-invasive prenatal aneuploid screening project in the market. However, non-invasive prenatal screening was first widely promoted in the United States, and the lack of FDA certification for genetic analyzers did not prevent the technology from being used clinically in the United States. In the United States, non-invasive prenatal screening programs are clinically accessible through the Reference Laboratories (CLIA) pathway, which is not subject to FDA restrictions. Under the CLIA policy, the government has strict quality control for these certified laboratories, and the laboratory management personnel have the corresponding licenses. If these two are qualified, the laboratory can legally charge and provide their own verified clinical services. At present, there is no reference laboratory policy like the United States in China, and only the product registration path can be taken. The author believes that the legal risks encountered in non-invasive prenatal screening in China are mainly caused by the differences in the medical regulatory systems between China and the United States, and not caused by the validity and safety of the devices and reagents themselves. The emergence of such problems is the direct manifestation of the domestic medical supervision system lags behind the new biotechnology innovation. The “Regulations on Strengthening Supervision†has been fully prepared and prepared by the CFDA and other regulatory agencies. The first is to issue a “Classification Definition Notice†for the registration of genetic testing related instrument reagents and algorithm software, for related products. The registration process is guided by the second; the second is the “Special Approval Noticeâ€, which can be used as a shortcut for the registration of innovative medical devices such as genetic analyzers. It will save time for the relevant clinical projects of genetic testing companies to obtain legal status as soon as possible. With energy. The author believes that the CFDA's supervision of the gene sequencing industry, especially the non-invasive prenatal screening program, will have a major impact on the industry, and both the promotion and the shuffling effect will be combined. If this policy is implemented, it will have a certain impact on the industry in the short term, but it will also bring out the real strength of the company in the industry. The enterprises that have obtained the registration certificate or license will obtain legal status and accelerate the market promotion. Competition will enable more and better clinical projects to serve the public. At the same time, strict market supervision will also form a certain protection for market pioneers and form certain policy barriers for the industry's latecomers. In short, in the long run, the promulgation of the “Regulations on Strengthening Supervision†is good news for the industry, left-handed strikes, right-handed release, and regulation of the industry, and will also prompt the industry to embark on the fast track of healthy development. The author is only shallow, the words of a family, only for the use of bricks and jade, welcome everyone to correct me!
Source: healthib club
With low-power, miniature size, the serial communication department range sensor with mm accuracy can measure up to 20m, 40m, 60m, 100m, 150m, 200m.
If you want to connect our laser range sensor boards together with your computer, you can directly choose the model with one USB connector.
In your project, you can use our distance sensor panel to measure a distance.
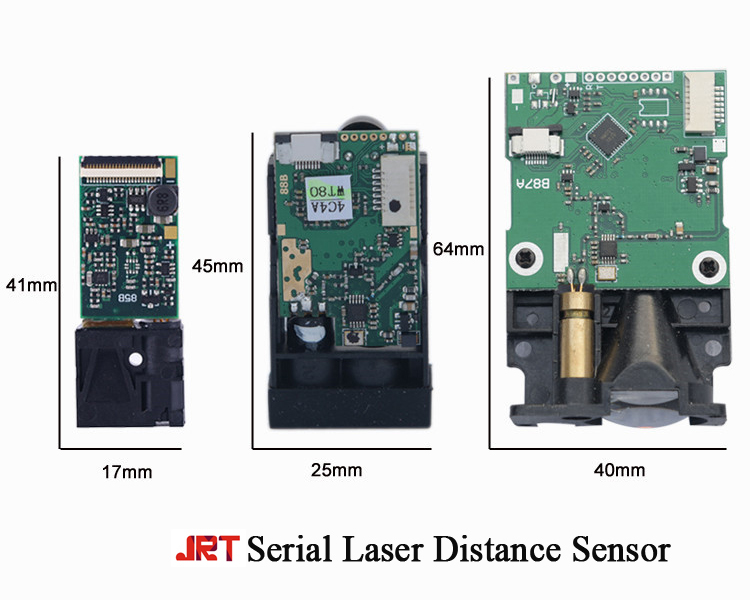
Serial Laser Distance Sensor,Serial Communication Laser Distance Sensor,Laser Range Sensor Boards,Distance Sensor Panel
Chengdu JRT Meter Technology Co., Ltd , https://www.rangingsensor.com