Research on new anticancer drugs is currently facing significant challenges, as only 5% of the compounds that showed efficacy in preclinical studies continue to be developed and become licensed drugs. Traditional 2D cell culture models are used to evaluate drug candidates in the early stages of the drug discovery process. However, there is increasing evidence that cells grown in two-dimensional monolayers do not accurately reflect the biological complexity of tumors. .
The need for better in vitro models compatible with high throughput screening has led to the development of 3D cell culture models, especially multicellular microspheres, which will retain many of the morphological and hereditary traits of tumors. In in vitro experiments, 3D culture models are widely considered to be more physiologically relevant models than 2D forms of culture systems. The 3D model more accurately reflects the complexity of the in vivo microenvironment and is used in many research areas such as oncology [1], hepatotoxicity [2], neurobiology [3], pancreatic research [4], Nephrology [5], and stem cell research [6]. These studies have subverted our understanding of cell behavior in in vitro culture and in vivo. However, due to the limitations of cell culture techniques, high-throughput screening with 3D culture systems has been very slow. Technical bottlenecks include experimental heterogeneity, low throughput, difficulty in automating, and costly.
Here, we describe cell sphere assays for 3D culture by two methods: 1) in ultra-low attachment plates; 2) on semi-solid agar. Both methods can be performed on 96 and 384 well microplate formats.
We then used Acumen hci laser scanning technology for fast full-plate image acquisition (5 minutes/block), giving a range of parameters such as the number, area and volume of spheres. Acumen hci is a perfect system for high-content analysis of microspheres. Its unique full-hole scanning capability captures information on all microspheres at different locations in a single well, while a scanning mirror with a certain depth of field makes it possible to The spheres in each layer are also captured at one time, and the volume of a single sphere is counted without Z-layer tomography, and data is collected multiple times.
method:
A: Formation of tumor microspheres in ultra-low adhesion plates
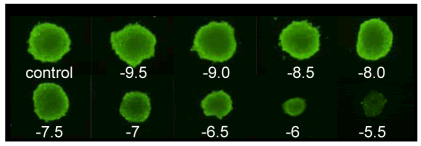
Corining's ultra-low adhesion panels feature opaque walls and a clear, rounded bottom. A proprietary ultra-low cell adhesion coating with hydrophilic, bio-inert and non-degradable properties by covalent attachment to the inner surface of the bottom of the well. This unique design on the bottom of the culture well allows 3D cell spheroid cultures to grow highly reproducibly. The opaque sidewalls and the proprietary bottom mesh design greatly reduce the crosstalk between the fluorescent, luminescent background and the holes and holes. In the well, the spheroids can be generated, cultured, and detected for fluorescent or luminescent signals without the need to transfer the spheroids. Currently, Corning ultra-low adhesion sheets are available in 96- and 384-well plates, both of which are easy to automate. a) Single cells of hepatoma cell line HepG2 suspended in complete medium were plated in Corning ultra-low adhesion plate (#4520&3830), plated density was 96-well plate, 200 μl per well contained 300 cells, each in 384-well plate 50μl of well contains 300 cells b) Within 24 hours, the suspended cells will assemble into a single 3D microsphere and precipitate in the center of the cell, as shown in Figure 1A on the right. c) After 72 hours, replace 50% of the medium with fresh medium containing 2 times the concentration of Doxorubicin (doxorubicin). The final concentration after replacement is 316pM~3.16μM. d) The formed microspheres continue to culture for 6 days |  Figure 1A |
B: Formation of tumor microspheres in soft agar
96-well plates | 384-well plate |  Figure 1B | |
Basal layer (medium containing 0.6% agar) | 25μl | 10μl | |
Cell layer (single cell suspension containing 0.4% agar) | 50μl contains 500 cells | 20μl contains 150 cells | |
Medium cover | 75μl | 30μl | |
a) Preparation of soft agar according to the above system b) Incubate soft agar containing single cells in a 37 ° incubator c) After 24 hours, add 5 μl of Doxorubicin (doxorubicin) to the well at a final concentration of 1 nM to 1 μM. d) continue to culture the microspheres for 4 days, and replace the overlay medium containing doxorubicin for 3 days. | |||
C: staining and image acquisition
| a) Add the dye Calcein-AM (final concentration 5 μM) to the wells containing the microspheres of the cells to stain the tumor microspheres formed by the two methods, and incubate for 1 hour at 37 degrees after the dye is added. b) The board then uses Acumen for image acquisition (5 minutes for image acquisition and data output per board) c) The data is analyzed online and obtained while scanning the image. The resulting report contains information on the number, area and volume of the sphere, fluorescence intensity, etc. |  Figure 1C |
result:
Tumor microspheres grow in ultra-low adhesion plates
A 96-well plate | |
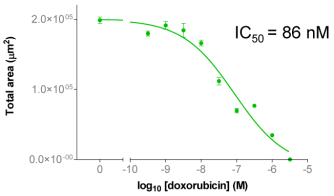
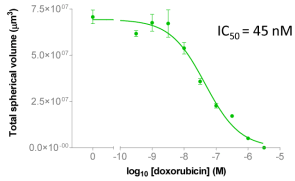
 | Figure 2A: Full-hole TIFF image of HepG2 tumor microspheres grown in 96-well ultra-low adhesion plates, the bottom of which shows the concentration of doxorubicin added to each well. 2B-C: drug dose-effect curve (mean±SEM, n=6) |
B | C |
 |  |
A 384-well plate | |
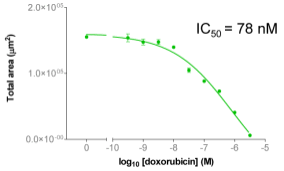
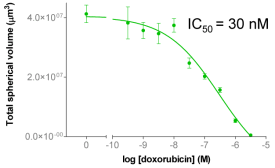
 | Figure 3A: Full-hole TIFF image of HepG2 tumor microspheres grown in 384-well ultra-low adhesion plates, the bottom of which shows the concentration of doxorubicin added to each well. 3B-C: drug dose-effect curve (mean±SEM, n=8) |
B | C |
 |  |
Tumor microspheres grow in soft agar
A | B | C |
 | 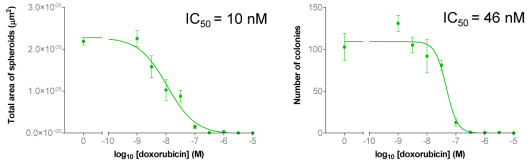 | |
D | ||
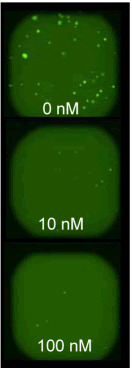
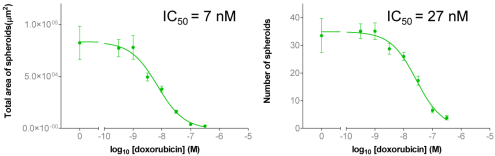
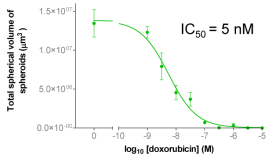 | Figure 4A: Full-hole TIFF image of HepG2 tumor microspheres grown in 96-well soft agar, showing the concentration of doxorubicin added to each well. 4B-D: drug dose-effect curve (mean±SEM, n=3) | |
A | B | C |
 |  | |
D | ||
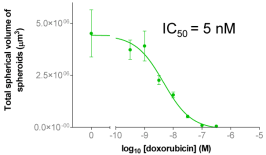 | Figure 5A: Full-hole TIFF image of HepG2 tumor microspheres grown in 384-well soft agar, showing the concentration of doxorubicin added to each well. 5B-D: drug dose-effect curve (mean±SEM, n=4) | |
to sum up:
The cell population, different cell types and micro-tissue structures growing in the 3D culture system coordinate and interact with each other, simulating the real living environment of the living substance in the micro-environment of the body, providing high quality for the researchers to analyze and overcome the disease. Data information. We established two simple and stable methods for 3D culture of tumor microspheres in 96 and 384 microplates. Information on number, area, volume and fluorescence intensity was obtained by staining and image acquisition. The consistency of the drug dose-effect curve reflects the reliability and reproducibility of the two methods. These data indicate that the acumen hci laser scanning system is a high-throughput (5 min/plate) of tumor microspheres obtained based on two culture systems. An ideal platform for analysis, which has been widely used in oncology [7-9] and stem cell research in the past [10-12].
References:
1. Maria V, Sharon G, Frances B, et al. Advances in establishment and analysis of three dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biology, 2012, 10:29
2. Patricio G, Nicola JH, Ute A, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol, 2013, 87(8): 1315-1530
3. Yinzhi L, Amish A, Ke Ch, et al. Neural Cell 3D Microtissue Formation is Marked by Cytokines' Up-Regulation. PLoS One, 2011, 6(10): e26821
4. Yesl J, Ah RK, Jae S L. 3D co-culturing model of primary pancreatic islets and hepatocytes in hybrid spheroid to overcome pancreatic cell shortage. Biomaterials, 2013, 34(15): 3784-3794
5. Anna IA, Brenda KM, Glen DP, et al. A 3-D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials, 2012, 33(18): 4700-4711
6. Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell, 2013, 12(5): 520-530
7. Weijuan W, Chen Bi, Kelly MC, et al. Inhibition of tumor growth and metastasis in non-small cell lung cancer by LY2801653, an inhibitor of several oncokinases, including MET. Clin Cancer Res, 2013, 19(20): 5699–5710
8. Kai W, Ho YL, Shephanie S, et al. Genomic Landscape of Copy Number Aberrations Enables the Identification of Oncogenic Drivers in Hepatocellular Carcinoma. Hepatology, 2013, 58(2): 706-717
9. Shane RH, Jeremy T, Anthony PO, et al. An HTS-Compatible 3D Colony Formation Assay to Identify Tumor-Specific Chemotherapeutics. J Biomol Screening, 2013, 18(10): 1298-1308
10. Koppany V, Hydeyuki O, Robert D, et al. A molecular screening approach to identify and characterize Inhibitors of glioblastoma stem cells. Mol Cancer Ther, 10(10), 1818-1828
11. Anne D, Jimmy E, Richard JS, et al. CXCR4 Expression in Prostate Cancer Progenitor Cells. PLoS One, 2012, 7(2): e31226
12. Claudia P, Ina K, Leoni K. Discovery of the cancer stem cell related determinants of radioresistance. Radiother Oncol, 2013, 108(3), 378-387
Crab Stick,Crab Surimi,Half Cut Swimming Crab,Frozen Surimi Crab Stick
Zhejiang Zhoushan Jiaze Aquatic Co., Ltd. , https://www.tianjia-aquatics.com