Bile acids are a group of biochemical compounds that belong to the large family of steroids. While steroids have gained a negative reputation in recent decades due to their association with cardiovascular diseases, sports doping, or misuse of hormone and corticosteroid medications, they play essential roles in the body.
Steroids, especially those based on cholesterol, are vital for cell function. Their roles include regulatory, structural, and metabolic functions, as well as acting as precursors for other important molecules. As fine chemical suppliers, we aim to provide an overview of bile acids, highlighting their structure, properties, and biological significance.
Structure of Bile Acids
As mentioned earlier, bile acids are a type of steroid. Their molecular structure consists of a four-ring system known as cyclopentane-perhydrophenanthrene, or "sterane." This framework is the foundation for all steroid molecules.
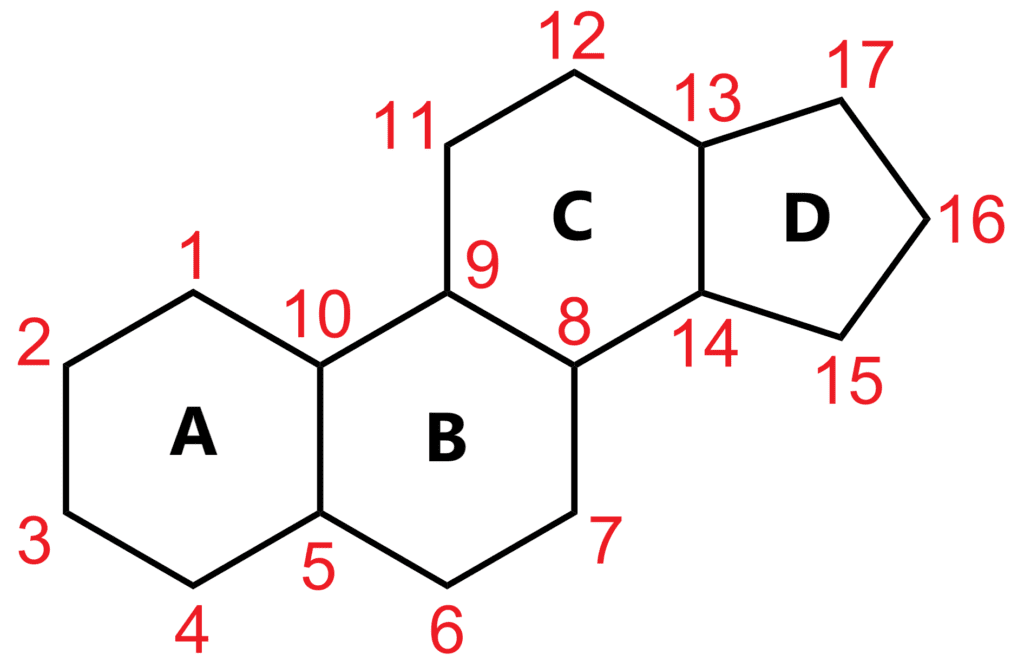
The carbon skeleton of sterane, the simplest steroid. The numbering follows IUPAC guidelines.
In terms of conformation, the A and B rings are typically in a cis configuration, while the B/C and C/D rings are trans. The A ring can adopt either a chair or a boat (less stable) conformation depending on stability requirements.
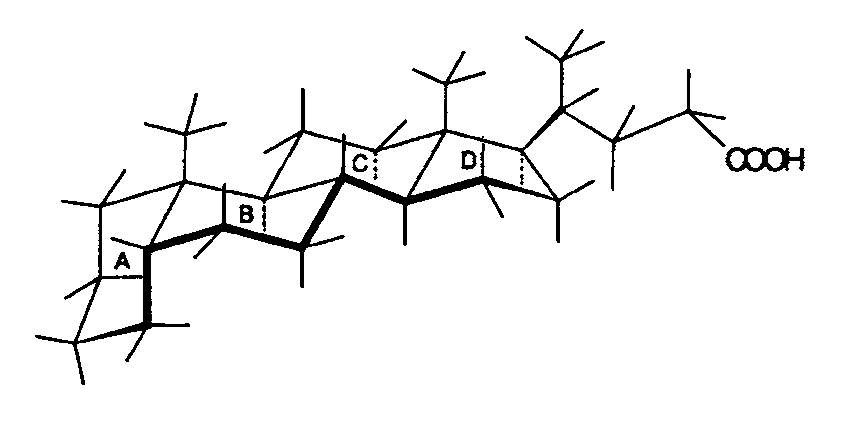
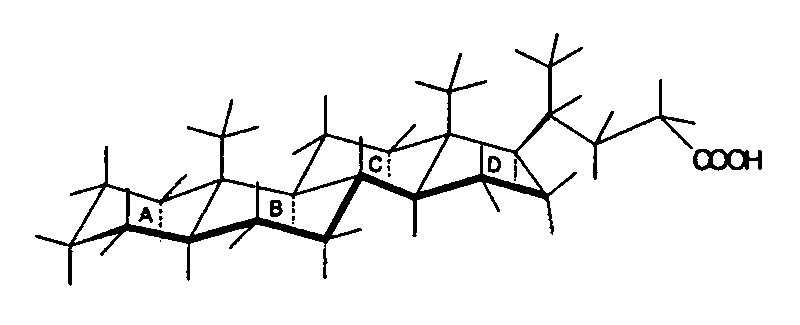
Structure of 5α-cholanic acid (left) and 5β-cholanic acid (right), showing how different conformations affect the molecule.
Hydroxyl groups are commonly found in the A, B, and C rings, particularly at positions C3, C6, C7, C12, and C23. Methyl groups are also frequently present at C10 and C13.
Stereochemistry plays a crucial role in the behavior of these molecules. The hydrogens at C5 and C8 are in a cis configuration, while those at C9 and C14 are trans.
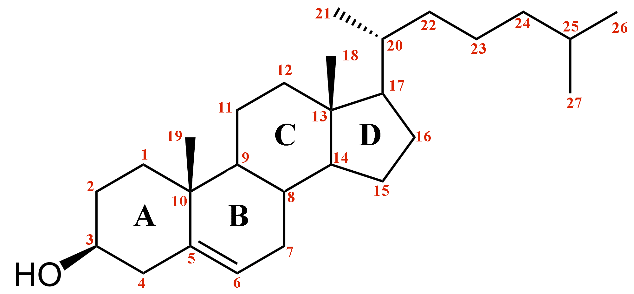
Cholesterol's structure with carbon numbering.
At the C17 position, there is usually an aliphatic chain with varying degrees of functionalization. The length and functionality of this chain, along with the presence of methyl and hydroxyl groups and stereochemistry, define the unique properties of each steroid.
Biosynthesis and Classification
The basic bile acid in nature is cholanic acid, from which various bile acids are derived through hydroxylation of different carbon positions. However, it is rarely found in mammals.
Instead, cholesterol serves as the main source. Although cholesterol is a highly hydrophobic molecule, it is a major component of cell membranes. When the end of its aliphatic chain is oxidized, it becomes a bile acid, a process carried out by liver cells, resulting in primary bile acids like cholic acid and chenodeoxycholic acid:
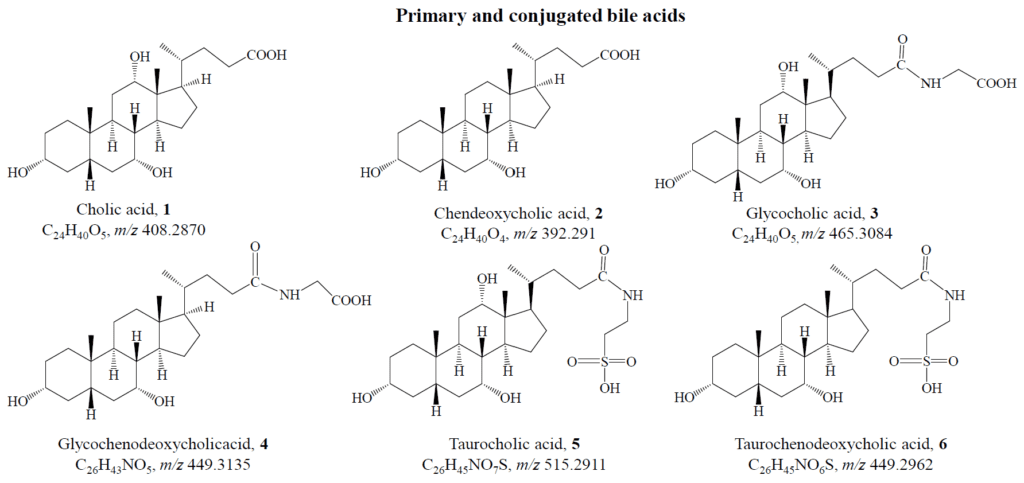
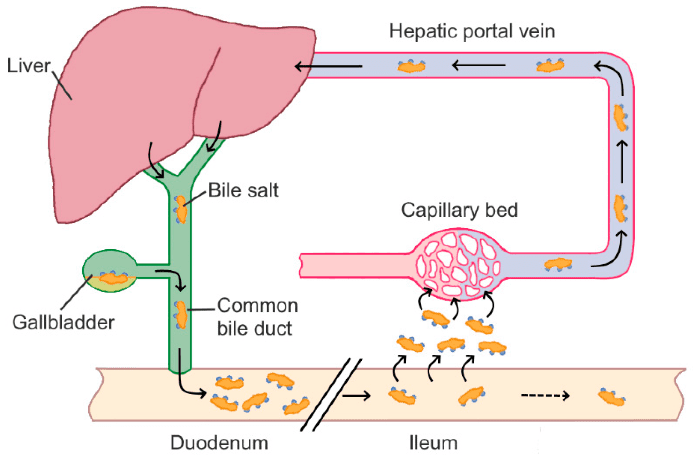
These acids travel to the small intestine via the gallbladder, where the environment is slightly acidic (pH 5–6.5). However, their pKa values range between 3 and 5, meaning they are mostly protonated under these conditions, making them poorly soluble in water. To overcome this, the liver conjugates them with glycine or taurine, forming ionic complexes that enhance solubility.
Once in the gut, intestinal bacteria break down these complexes and convert the primary bile acids into secondary ones, mainly through dehydroxylation at the C7 position. Further transformations, such as epimerization or conjugation with N-acetylglucosamine, may lead to tertiary bile acids.
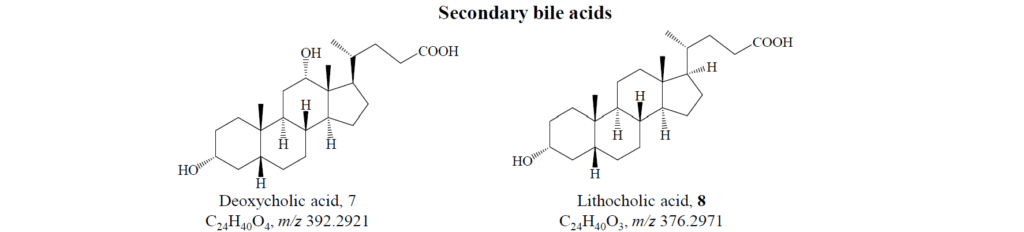
Some of these bile acids are reabsorbed in the gut and returned to the liver, completing what is known as enterohepatic circulation:
Functions of Bile Acids
Bile acids exhibit surface-active properties in neutral or basic environments. The aliphatic chain, containing a carboxyl group, acts as the hydrophilic part, while the rest of the molecule is lipophilic. This makes them excellent emulsifiers, helping to break down fats and non-polar substances into micelles for easier absorption by the small intestine.
Beyond emulsification, bile acids act as signaling molecules by binding to various receptors, triggering important biochemical processes such as inflammation regulation and calcium mobilization. They also influence protein kinase C activation, cyclic AMP synthesis, and pro-inflammatory cytokine secretion.
Additionally, bile acids participate in mitochondrial oxidative processes, insulin receptor signaling, and the regulation of cholesterol levels. They also aid in the elimination of waste products like bilirubin.
In summary, bile acids have multiple critical roles in the body. While cholesterol has long been seen as a risk factor, it is clear that it is essential when present in appropriate amounts. Managing intake and maintaining balance is key to avoiding related health issues.
Â
Â
References:
- Kuhajda, K. et al., “Structure and origin of bile acids: an overviewâ€, European Journal of Drug Metabolism and Pharmacokinetics 2006, Vol. 31, No. 3, pp. I3S-143
- Chiang, J.Y.L., “Bile acids: regulation of synthesisâ€, Journal of Lipid Research, Volume 50, 2009, 1955–66.
- Singh, J. et al., “A Review on Bile Acids: Effects of the Gut Microbiome, Interactions with Dietary Fiber, and Alterations in the Bioaccessibility of Bioactive Compoundsâ€, J. Agric. Food Chem., Just Accepted Manuscript • DOI: 10.1021/acs.jafc.8b07306
Orthopedic Skills,Fixed Orthodontic Model,Natural Large Skull Model,Male Torso Anatomy Model
Yinchuan Erxin Technology Co., LTD , https://www.exmedmodel.com